
Oxygen is not easily soluble in water, and fermentation broth and microbial metabolites in laboratory fermenters can even reduce the solubility of oxygen in the fermentation process. Therefore, controlling DO is not only to grow the beneficial metabolites in fermentation, but also to make the experiment reduce the cost and increase the efficiency.
Laboratory fermenter DO electrode working principle
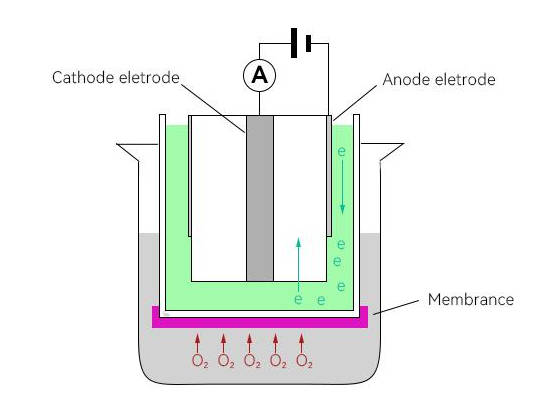
The common polarographic dissolved oxygen electrode generally consists of anode, cathode, oxygen permeable film and electrolyte. The oxygen permeable film is wrapped around the outer surface of the cathode. The material is generally PTFE, PVC, polyethylene, silicone rubber and other breathable materials.In addition, compared to primary cell electrodes, polarographic electrodes require an additional polarization voltage of 0.5 to 1.5 V in the cathode and anode. HOLVES laboratory fermenters come standard with METTLER TOLEDO dissolved oxygen electrodes, where durability and measurement accuracy are the main considerations.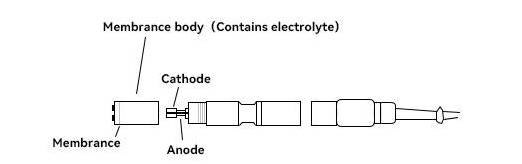
The oxygen is separated by electrolysis as it passes through the film to the surface of the cathode during use, and the released electrons form a current in the electrolyte.Because the content of dissolved oxygen through the membrane is proportional to the dissolved oxygen content in the water, the intensity of the current formed in the electrolyte is different for different dissolved oxygen content, and the current intensity can be monitored by electrodes.The current intensity monitored by the electrodes can be converted to a specific oxygen concentration according to Faraday's law, and the final value is obtained by compensating for temperature and air pressure.
How to adjust the DO of laboratory fermenter
In the process of fermentation, the relationship between DO concentration and other parameters of the process is relatively complex and influenced and constrained by multiple factors in the fermenter.The DO is controlled so that it can be stabilized within the expected value.We usually control the DO in the fermenter by aeration, agitation and replenishment.
Taking HOLVES laboratory fermenter as an example, users can set DO with STIR control and FEED control in multi-level cascade, then through stable RS-485 communication, the fermentation system HF-Control will receive the electrode transmission data in real time and take PID to regulate the relevant parameters step by step to reach ,and stabilize within the set DO value.
This article takes you to understand how to control the dissolved oxygen in laboratory fermenters, and also mentioned above that the dissolved oxygen is affected by many factors, and the temperature factor is one of them. So the next article will explain the temperature control of laboratory fermenters.






